V-Tech Injectable Omeprazole
For many years the gold standard of treatment for Equine Gastric Ulcer Disease, in South Africa, has been oral, enteric-coated omeprazole at a dose of 2 – 4 mg per kilogram once daily. It is well-documented and researched that oral omeprazole is most effective if given on an empty stomach at least 30 minutes before feeding. Since 2017 numerous studies undertaken by multiple research groups have shown positive results against acid suppression and improvements in gastric ulcer scores among horses treated with a once-per-week, long-acting, intramuscular omeprazole injection.

V-Tech released a compounded, long-acting omeprazole formulation in 2022. My colleagues and I, from Cape Vet Equine Practice, undertook a small in-practice case study to determine the efficacy of this formulation. V-Tech was involved in this case study, covering the cost of a repeat scope for 8 horses treated with the injectable omeprazole. The results of the case study were presented in a poster format at the World Veterinary Association Congress in Cape Town in April 2024.
Fifteen horses were presented to Cape Vet for gastroscopy over eighteen months, for a multitude of reasons: most notably, weight loss and changes in behaviour. Other complaints included girthing pain, poor performance and a dull coat. Each horse was subjected to two gastroscope procedures, performed under standing sedation, using a 3m flexible video-endoscope. The presence, or absence, of gastric ulcers on both the squamous and glandular mucosa was recorded, and the images were graded by two veterinarians. The horses were diagnosed with either squamous (15/15), or squamous and glandular lesions (11/11), and were treated with the compounded, injectable omeprazole at a dose of 4mg/kg, intramuscularly once weekly for a minimum of 4 weeks. Concurrent treatment with sucralfate at 12mg/kg, twice daily, orally as either a suspension or tablet formulation was used in 12 of the 15 cases. Each of the fifteen horses received a second scope, 5 – 10 weeks (average 7 weeks), after the start of treatment, to determine the efficacy of the treatment.
EGGD = Equine Glandular Gastric Disease
Table 1: Below: Data of the subject horses
|
Total number of horses in the study |
|
Diagnosed with ESGD |
|
Diagnosed with ESGD and EGGD |
|
Age |
|
Mares-gelding-stallions |
|
Thoroughbreds (pure or cross) |
|
Warmbloods |
|
Other breeds (Boerperd, Arab) |
|
Showjumping |
|
Dressage |
|
Endurance |
|
General purpose/hack, Eventing and Hunting |
|
Days to re-examination (range) |
|
Previous Treatment (oral omeprazole) |
|
15 |
|
15/15 (100%) |
|
11/11 (100%) |
|
Average 10.9 years old |
|
Mares 6 (40%), Geldings 9 (60%) |
|
8(53%) |
|
3(20%) |
|
4(27%) |
|
6(40%) |
|
2(13%) |
|
2(13%) |
|
5(33%) |
|
35-75days (Average 50.8 days/ 7.25weeks) |
|
7(47%) |


RESULTS:
The pylorus was not visualized in 36% of the horses and they were excluded from the glandular grading. Of the 11 horses with glandular lesions, 18% of the horses’ lesions resolved completely, while 82% showed marked improvement. No glandular lesions deteriorated or stayed the same.
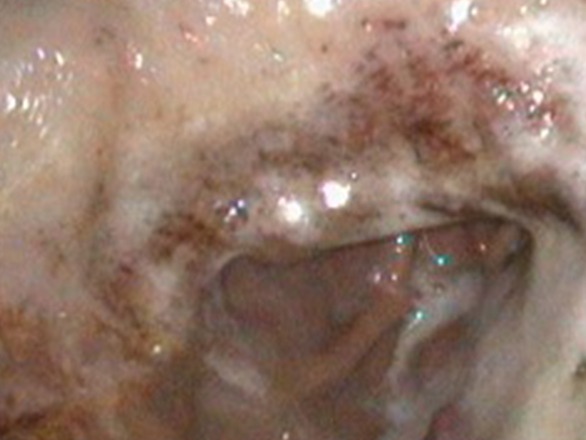
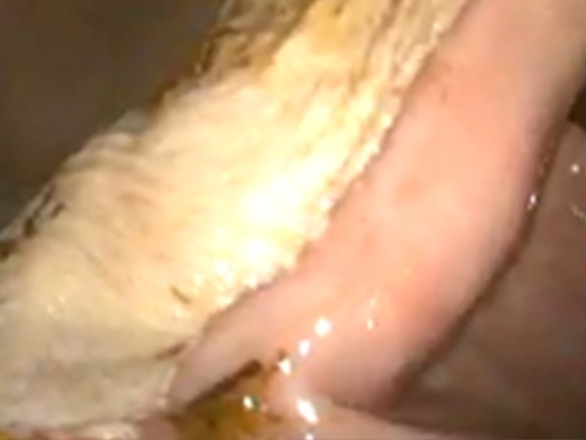
Figure 1: Severe, grade 4 squamous lesions before treatment.
Figure 2: Same horse after 7 weeks of injectable omeprazole
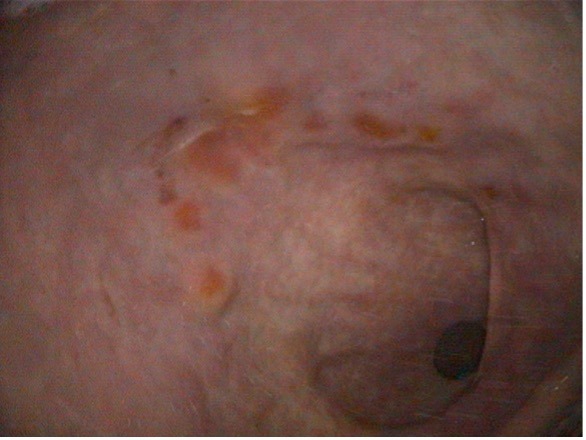
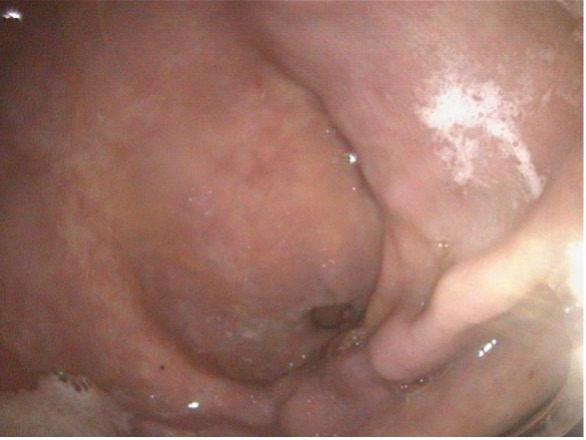
Figure 4: Glandular mucosa of the same horse after 6 weeks of injectable omeprazole
Difficulties and adverse reactions encountered during data collection included:
- Inadequately starved horses
- Load shedding
- Localised pain and swelling at the injection site.
- Thickness of the product and difficulty of injecting
CONCLUSION:
The use of the V-Tech compounded, injectable, intramuscular, omeprazole formulation for the treatment of ESGD and EGGD resulted in either resolution or a marked improvement in both squamous and glandular gastric lesions as well as clinical signs.